
altona Diagnostics is aware that the European Commission has realeased a proposal in January 2024 affecting the Regulation (EU) 2017/745 to extend the transition periods of IVDD CE-marked products. We will update our website section “Approach to the IVDR” accordingly.
The IVDR and altona Diagnostics
The IVDR is fully applicable since May 26, 2022. altona Diagnostics is ready.
Learn more
Amendment to the IVDR
Extended transition periods for products CE-marked under the IVDD have been accepted by the EU: IVDD products can be placed on the market until May 26 of 2025, 2026 and 2027, respectively, depending on the IVDR risk classification of the product.
Learn more
altona Diagnostics product portfolio and the IVDR
AltoStar® and FlexStar® products will be fully IVDR compliant and be available as IVD products for the foreseeable future.
Learn more
Extended availability of RealStar® products
Most RealStar® products will be offered as IVDD products until the respective transition period according to IVDR risk classes ends. RealStar® RUO products will remain available beyond these dates.
Learn more
Steps to IVDR compliance
At all times, customers will receive IVD products from altona Diagnostics, which are fully compliant with the applicable regulations.
Learn more
FAQs
altona Diagnostics and the IVDR
Learn more
The IVDR
Laboratories all over Europe are affected by immense regulatory changes applying to diagnostic devices and laboratory-developed tests (LDTs). The in vitro diagnostic regulation (IVDR), (EU) 2017/746) entered into force in 2017 and has superseded the in vitro diagnostic directive (IVDD, 98/79/EC) with full applicability since May 26, 2022. However, prolonged transition periods were established for IVDD products.
The new regulation will head the diagnostic market towards an increase in transparency, comparability, robust results, and reliability, respectively. The combination of these improvements will be beneficial to patients, clinicians, and laboratory professionals alike.
altona’s stance
Among the changes introduced by the IVDR are, for example, the increased involvement of notified bodies, a new risk classification system, and enhanced post-market surveillance and transparency. Therefore, altona Diagnostics has forcefully combined capacities for years to develop products corresponding to the IVDR. We are converting our AltoStar® and FlexStar® products into IVDR compliant versions.
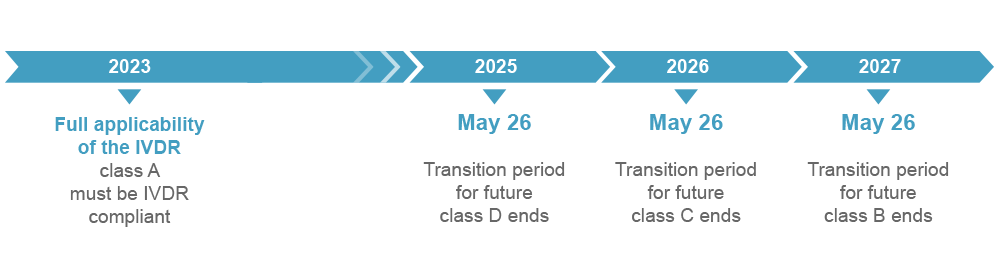
Extended transition periods
The diagnostic market faced major changes with the full applicability of the IVDR. In the advent of the tremendous shift, the European Commission composed a proposal to the IVDR in 2021. The suggestions were approved by the European Parliament and the EU Council. The proposal has led to an amendment to the IVDR, which provides updated transition periods.
Although the requirements of the IVDR remained unaffected, the following aspects apply with the publication of the Official Journal of the EU on January 28, 2022:
altona’s stance
As the consequences of the COVID-19 pandemic have intensively impacted all parts of the healthcare sector, altona Diagnostics perceives the transition periods of the amendment to the IVDR as positive, retaining both high-level quality and unrestricted availability of diagnostic products, e.g., PCR assay kits. Despite the extension of IVDR transition periods, altona Diagnostics has already taken action to convert our product portfolio to IVDR compliance.
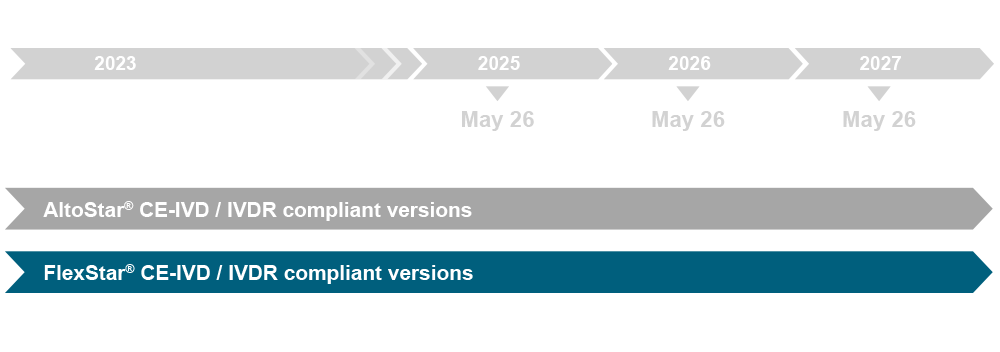
altona Diagnostics is committed to provide IVDR-ready products to our customers to facilitate early adoption of the new requirements. We have focused on the approach of launching AltoStar® products, which comprise a technical documentation that is prepared to meet the requirements of the IVDR. These products combine the AltoStar® Molecular Diagnostic Workflow with the usability of other workflows (manual or automated) and, in this way, are “open workflow” compatible.
The AltoStar® product line is already available as CE-IVD and all upcoming products of the new FlexStar® product line will also be available as CE-IVD. These products are aimed to be IVDR compliant in 2024 certified by the notified body. Given that the IVDR has gained full applicability by May 26, 2022, our products of the risk class A are already IVDR compliant. This applies to the AltoStar® Purification Kit 1.5, the AltoStar® Whole Blood Pretreatment Buffer 1.5, and the FlexStar® (RT-)PCR Amplification Mix 1.5.
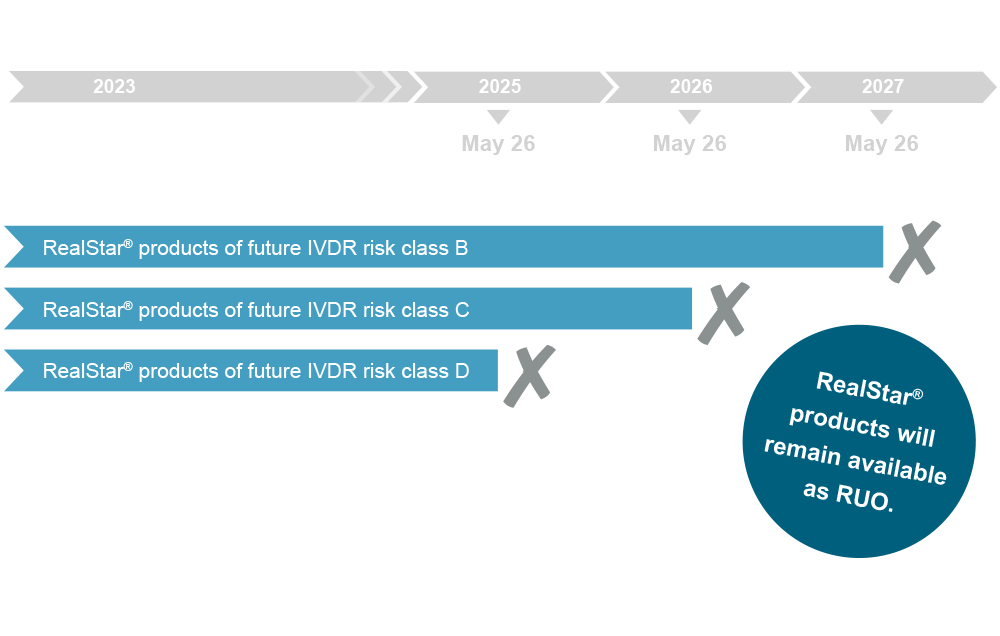
Following the IVDR amendment, RealStar® products will remain available as IVDD compliant CE-IVD products. Nevertheless, RealStar® products will not be released as an IVDR compliant version, in contrast to the AltoStar® and FlexStar® counterparts, but will be eventually discontinued. Hence, the last shipments of RealStar® CE-IVD products will be processed in line with the end of the applicable transition periods according to the respective IVDR risk class. altona Diagnostics kindly asks for your understanding that we are not yet able to provide a more precise discontinuation date for individual RealStar® CE-IVD products at this point. We will keep you posted and inform you about dates ahead of time. These dates will also be posted on this web page. However, RealStar® products will remain available as for research use only (RUO).
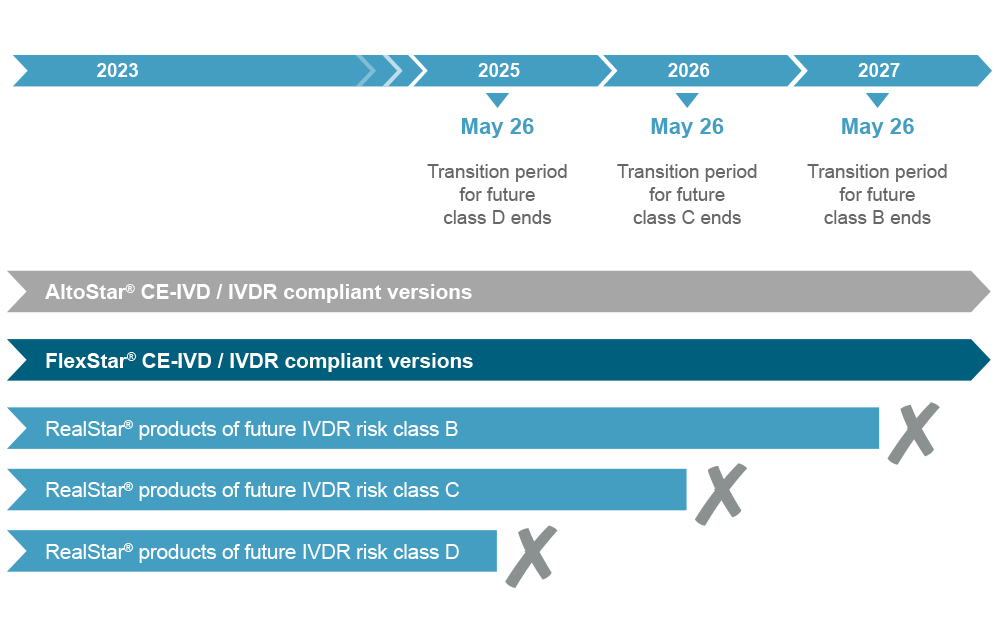
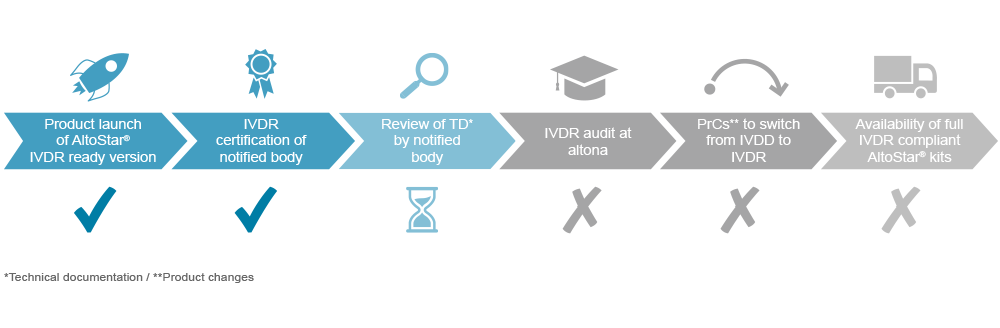
The successful transition of marketed devices to IVDR compliance requires dedicated and enduring preparation. altona Diagnostics initiated the process early to obtain AltoStar® products which will be compliant with the IVDR and cover the needs of customers who are not yet benefiting from the automated AltoStar® Workflow. These CE-IVD products are available for orders.
However, to achieve full IVDR readiness altona Diagnostics must rely on a multiple-step model. The majority of in vitro diagnostic devices have to be approved by the notified body under the IVDR. Since the notified body supervising altona Diagnostics was IVDR certified, the technical documentation of selected products is reviewed before an IVDR audit can be performed. We will be allowed to get certified to declare product conformity under the IVDR. With the achievement of that milestone, altona Diagnostics will be capable to conduct the necessary activities to eventually transform the CE-IVD devices into IVDR compliant versions. Nevertheless, customers can already benefit from our products ahead of the implementation of the switch to achieve full IVDR compliance.
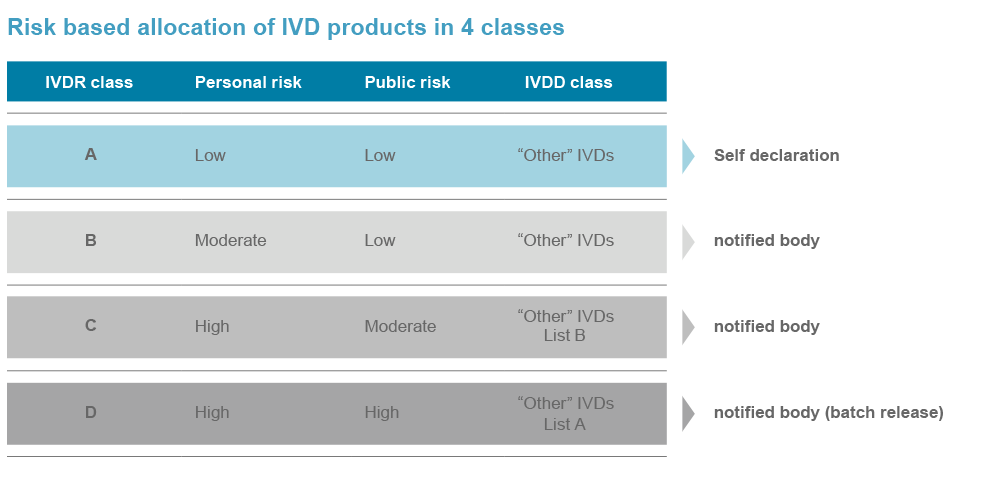
The IVDR gained full applicability by May 26, 2022. Devices, which belong to the IVDR risk class A, must be IVDR compliant since then. IVD devices, which are categorized to future IVDR risk classes B–D, benefit from transition periods of 3 to 5 years.
altona Diagnostics is committed to conduct the transition from IVDD to IVDR products for customers smoothly and quickly. However, the IVD regulation has certain impacts on the product portfolio:
For more detailed information concerning our products please contact your local altona Diagnostics sales representative.
The IVDR transition periods for IVDD products were extended according to the IVDR risk class.
*IVDR-compliant products versions already available.
The in vitro diagnostic directive (IVDD) was replaced by the in vitro diagnostic regulation (IVDR). This goes in line with the following major aspects:
Laboratory developed tests (LDTs), which are in-house developed and produced by healthcare institutions, fall within the scope of the IVDR. LDTs must fulfill the General Safety and Performance Requirements of the Annex I of the IVD Regulation since May 26, 2022, and additionally, the marketing and transfer of LDTs to other legal entities are no longer permitted. Thus, laboratories do have to demonstrate compliance with the Annex I. Further requirements (such as QM system, EN ISO 15189 compliance (accreditation of laboratory), provision of information to the competent authority, declaration of the fulfillment of Annex I, compiling the technical documentation) have to be fulfilled starting May 26, 2024. In addition, the justification of the use of LDTs (e.g., proof of uniqueness of the LDT) will be mandatory starting May 26, 2028.
If an adequate product is available for the diagnosis/treatment of a (rare) disease, the usage of commercially available PCR assays over LDTs will be highly recommended. That recommendation is underlined by powerful arguments providing multiple major advantages such as:
If laboratories still need to run LDTs (e.g., due to uniqueness of the LDT), altona Diagnostics can offer the FlexStar® LDT (RT-)PCR Detection Mix 1.5 RUO (see here for details) accompanied by the automation on the AltoStar® workflow to support consolidation on one workflow.
altona Diagnostics GmbH
Mörkenstr. 12
22767 Hamburg, Germany