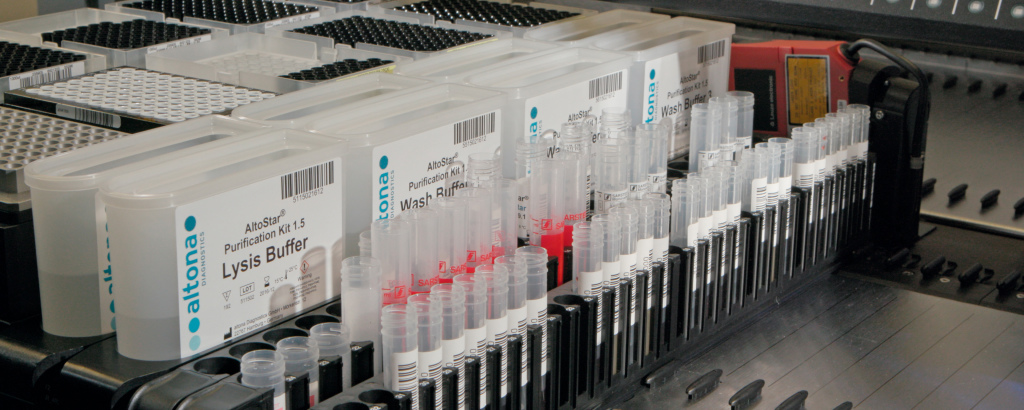
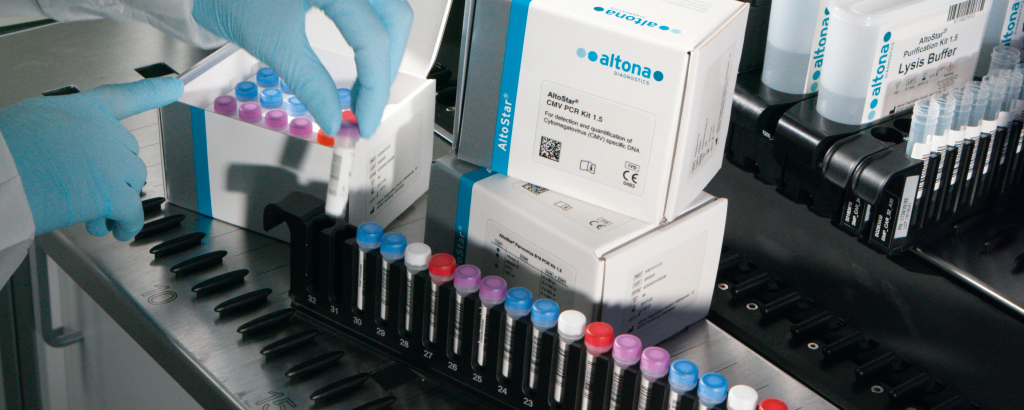
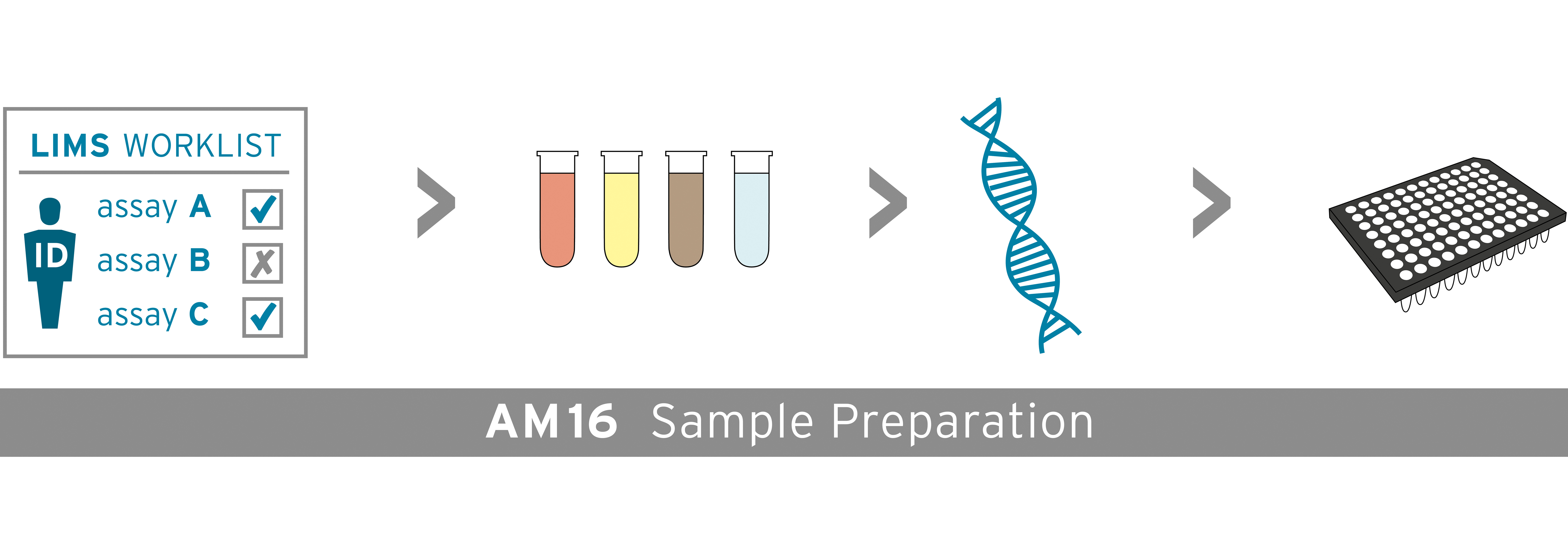
The AltoStar® Automation System AM16 is a robotic pipetting workstation used for sample purification and PCR setup controlled by the AltoStar® Connect software. It is intended to be used in combination with the AltoStar® Purification Kit 1.5, the AltoStar® Internal Control 1.5 and altona Diagnostics PCR kits for automated purification of nucleic acids and automated assay setup for in vitro diagnostic purposes.
The AltoStar® AM16 is intended for use by professional users trained in molecular biological techniques and the operation of the AltoStar® AM16 instrument.